Contents
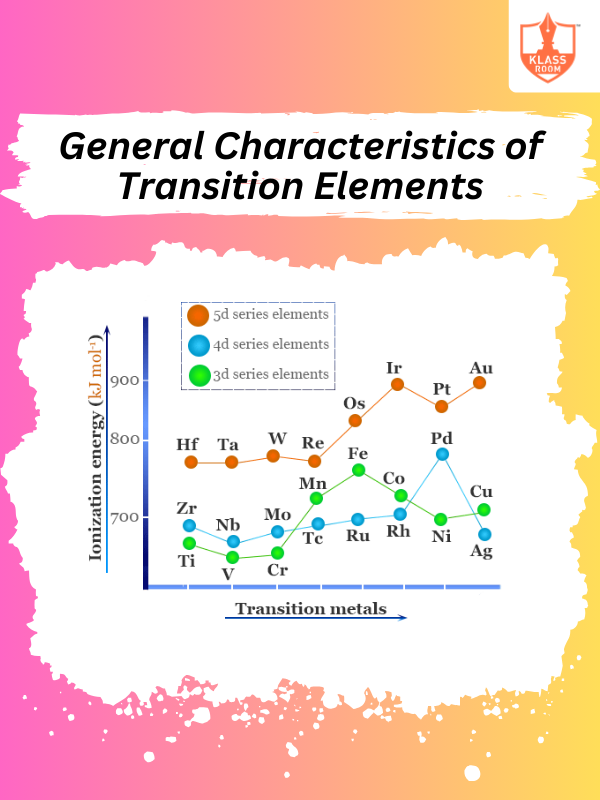
General Characteristcs of Transition Elements
Description: General Characteristics of Transition Elements include variable oxidation states, colored compounds, and catalytic properties.
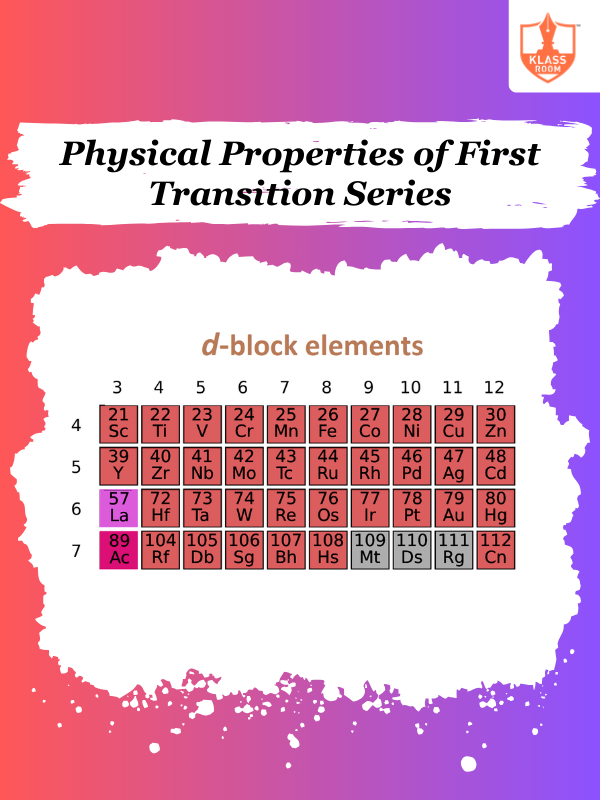
Physical Properties of First Transition Series
Description: Physical Properties of First Transition Series involve high density, melting points, and metallic conductivity.
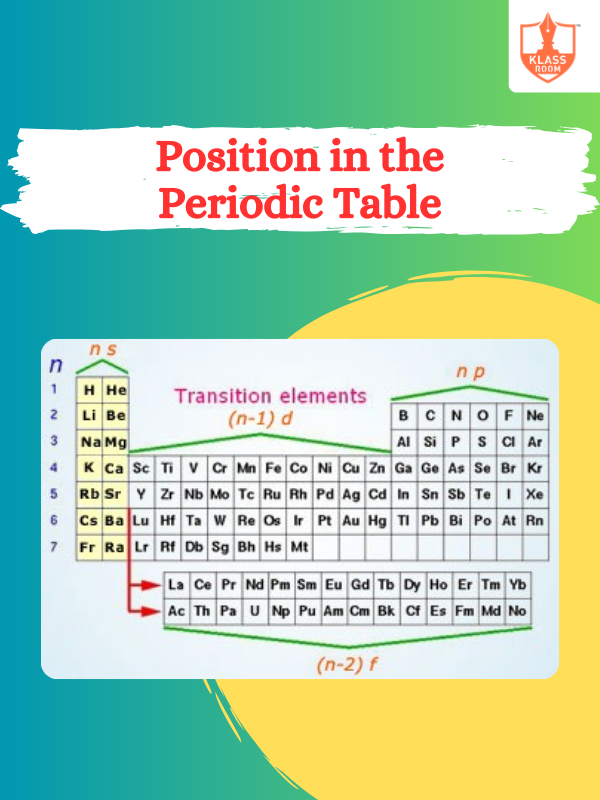
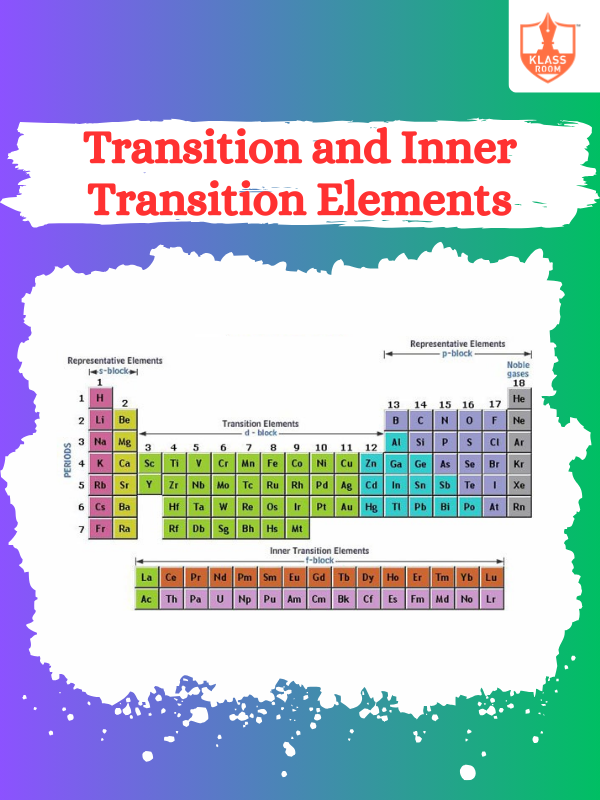
Position in the Periodic Table
Description: Position in the Periodic Table is determined by atomic number, influencing elements' properties and reactivity trends.
Color Properties & Alloy Formation
Description: Transition elements exhibit color due to d-d electron transitions and form alloys by metallic bonding.
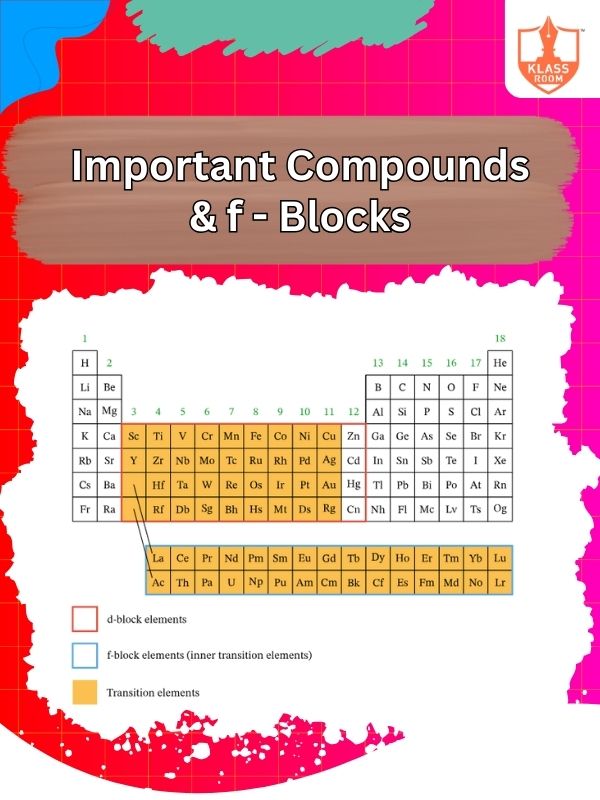
Important Compounds & f - Blocks
Description: f-block elements form colored compounds, exhibit variable oxidation states, and are used in nuclear and electronic applications.
Application of f - Block
Description: f-block elements have variable oxidation states, unique electronic configurations, and are applied in nuclear technology.