Contents

Thomson's & Rutherford's Model
Description: Thomson's model proposed electrons in a positive sphere while Rutherford's model introduced a central nucleus.

Bhor's Model of Hydrogen Atom
Description: Bohr's model describes hydrogen atom with quantized orbits, explaining energy levels and spectral lines.

Transition of Electron
Description: Transition of electron involves movement between energy levels, releasing or absorbing specific amounts of energy.

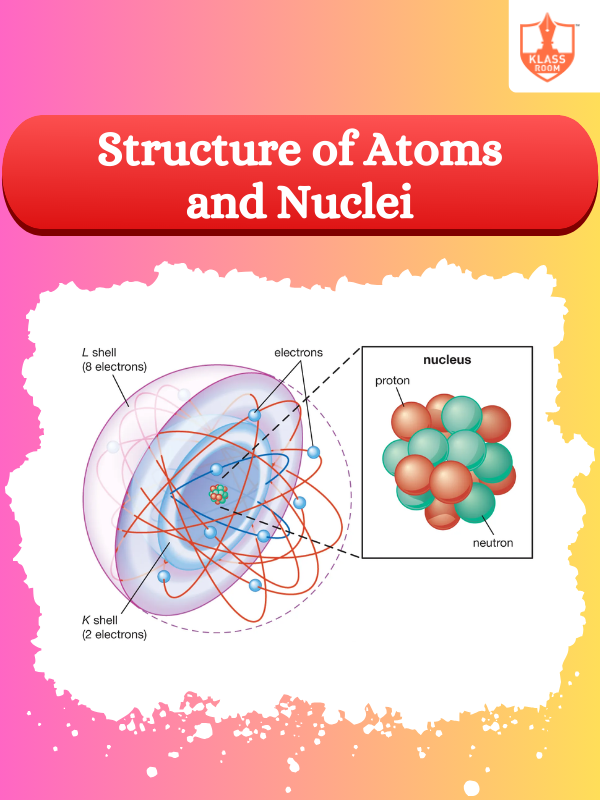
Constituent of Nucleus
Description: Constituents of nucleus are protons and neutrons, collectively known as nucleons, held by strong force.

Radioactive Decay
Description: Radioactive decay is the spontaneous emission of radiation from unstable nuclei in radioactive materials.

Radioactive Material
Description: Radioactive material emits ionizing radiation from unstable nuclei, undergoing spontaneous decay into stable forms.