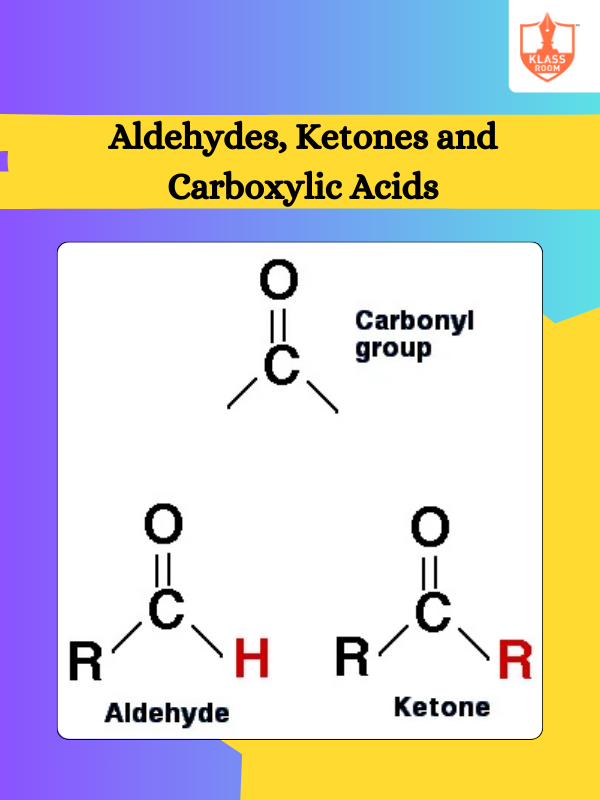
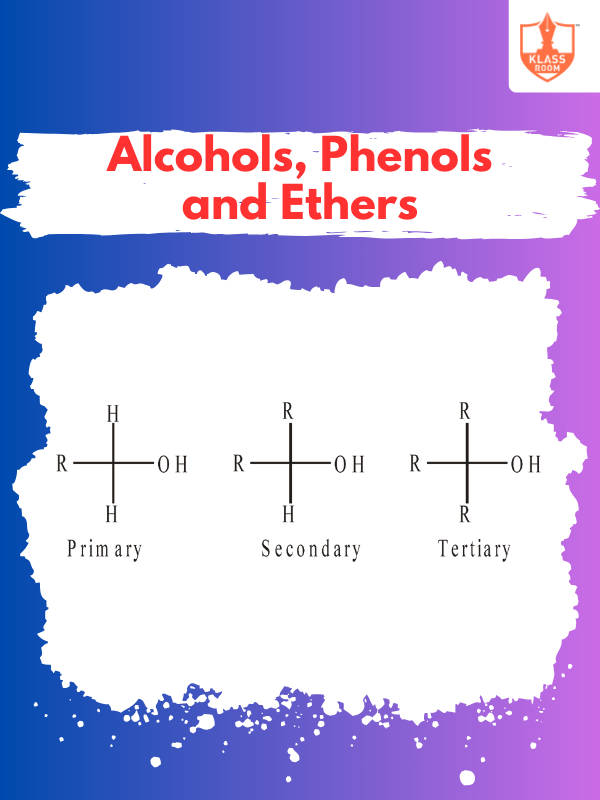
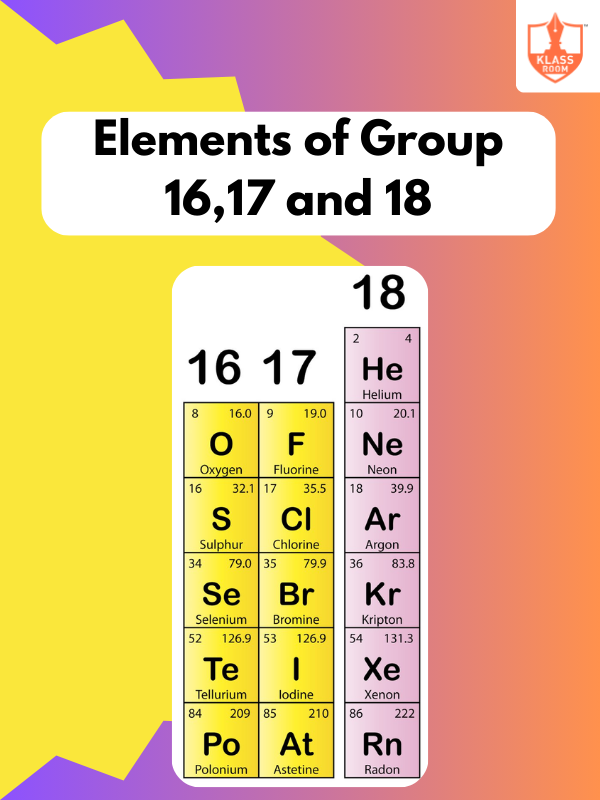
Contents
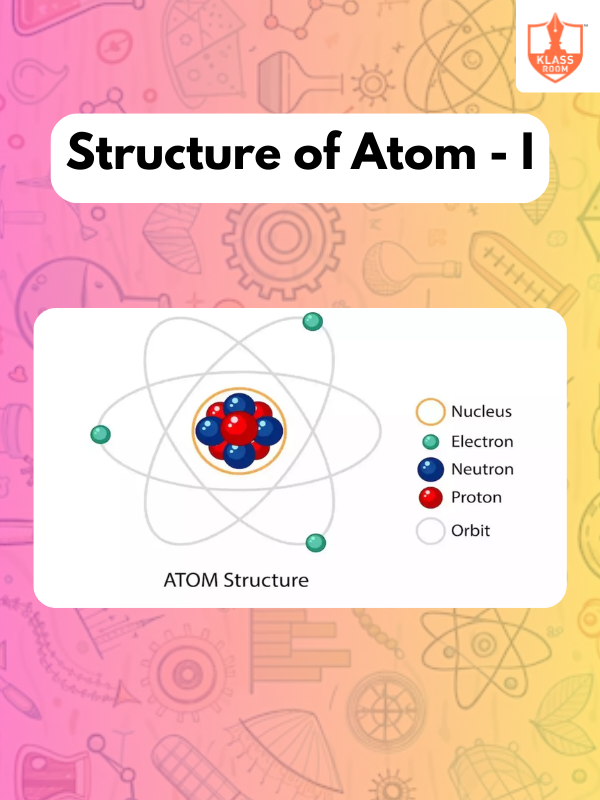
Structure of Atom - I
Description: Structure of Atom - I explores atomic models, subatomic particles, and the arrangement of electrons in atoms.
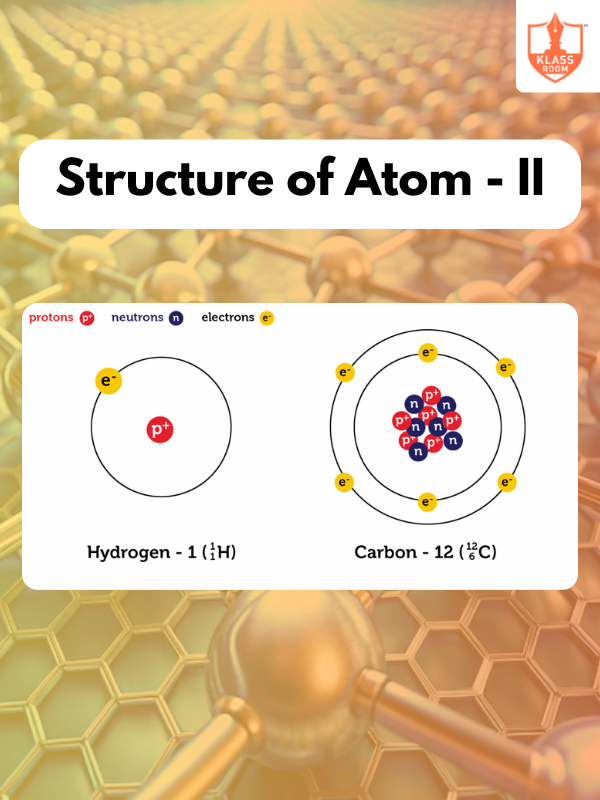
Structure of Atom - II
Description: Structure of Atom - II covers quantum numbers, electron configuration, and the behavior of electrons in atomic orbitals.
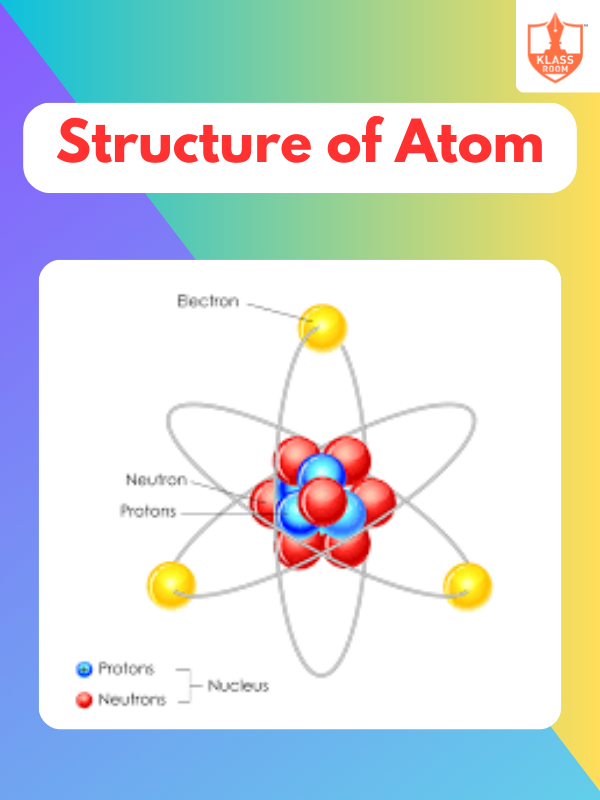


.png)