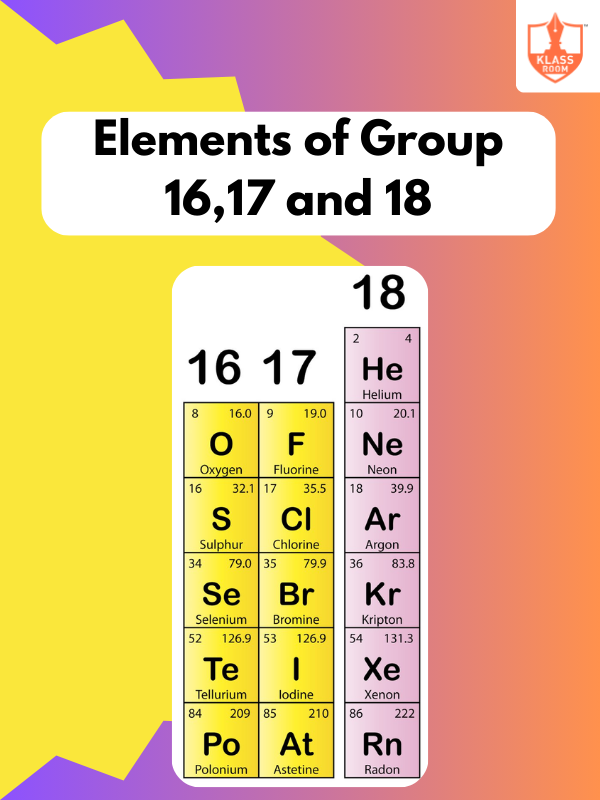
Contents
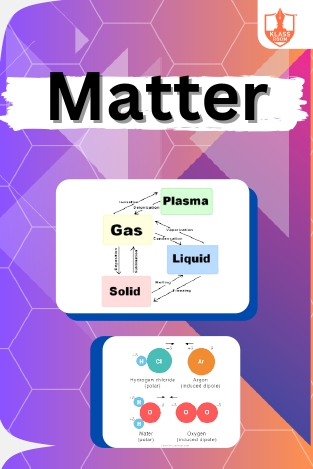
Matter
Description: Introduction to states of matter explains the different phases of substances. Conditions and intermolecular forces influencing these states are discussed, including dipole-induced dipole interactions, elucidating molecular behavior in different environmen
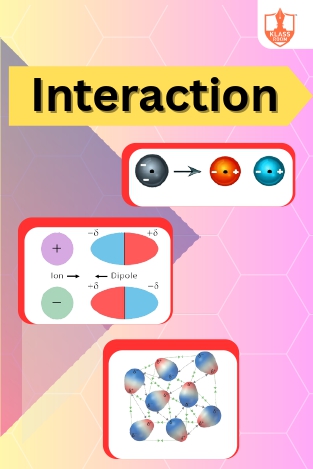
Interaction
Description: Ion-dipole interaction, non-polar interaction, and dispersion forces are intermolecular attractions affecting gases. These forces, along with measurable properties, elucidate the behavior and characteristics of gas molecules in various environments.
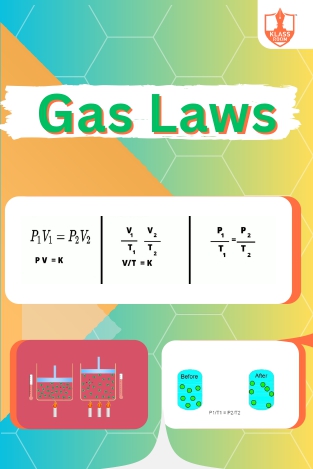
Gas Laws
Description: Gas laws govern the behavior of gases under different conditions. Charles’s law relates volume and temperature, while Gay Lussac’s law describes the relationship between pressure and temperature in a fixed volume.
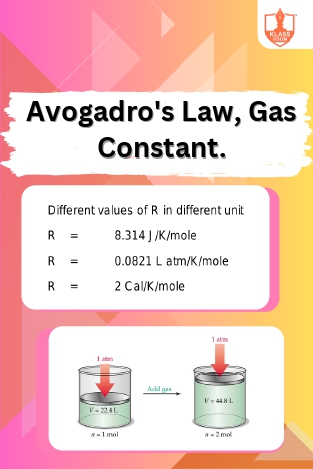
Avogadro's Law & Constant
Description: Avogadro's Law states that equal volumes of gases at the same temperature and pressure contain an equal number of molecules. The gas constant relates the properties of gases in ideal conditions.

Problems
Description: Problems Based on Ideal Gas Equation
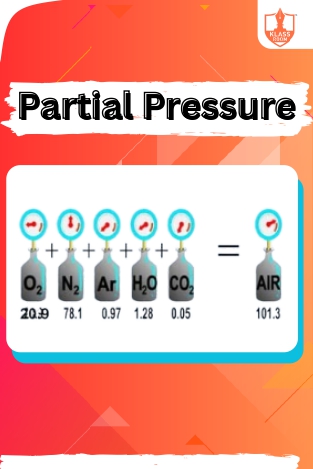
Partial Pressure
Description: Dalton's Law of Partial Pressure states that in a mixture of gases, the total pressure is the sum of the partial pressures of each gas. Problems based on Avogadro & Ideal Gas Law involve gas volume and quantity calculations.

Dalton's Law
Description: The application of Dalton's Law encompasses various scenarios where the total pressure of a gas mixture is determined by the sum of the partial pressures contributed by each individual gas component.

Ideal & Real Gases.
Description: Diffusion and effusion involve gas movement. Graham's law and partial pressure problems relate to gas velocities. Kinetic Molecular Theory explains gas behavior. Vander Waal's equation and its application elucidate deviations between ideal and real gases.
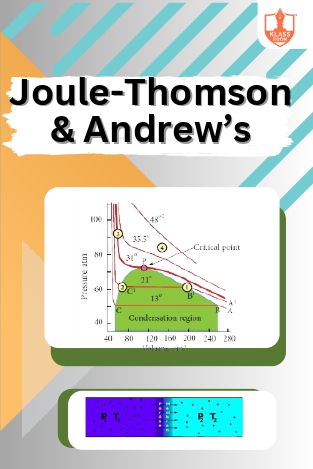
Joule-Thomson & Andrew’s
Description: The Joule-Thomson effect concerns temperature changes due to gas expansion. Andrew’s isotherm studies gas adsorption. Problems on liquefaction involve gas compression and cooling calculations.


.png)