Contents
Some Basic Concepts of Chemistry
Description: Some Basic Concepts of Chemistry introduces fundamental principles like atoms, molecules, moles, and chemical reactions.

Description: Some Basic Concepts of Chemistry introduces fundamental principles like atoms, molecules, moles, and chemical reactions.

Board: State Board
Stream: Science
Standard: XI
Course: Chemistry
Know More.png)



Board: State Board
Stream: Science
Standard: XI
Course: Chemistry
Know More
Board: State Board
Stream: Science
Standard: XI
Course: Chemistry
Know More
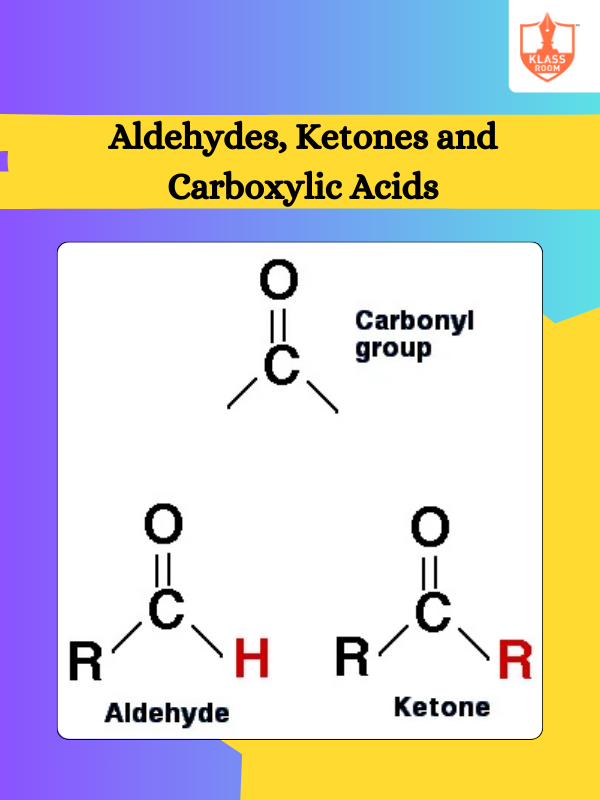
Board: State Board
Stream: Science
Standard: XII
Course: Chemistry
Know More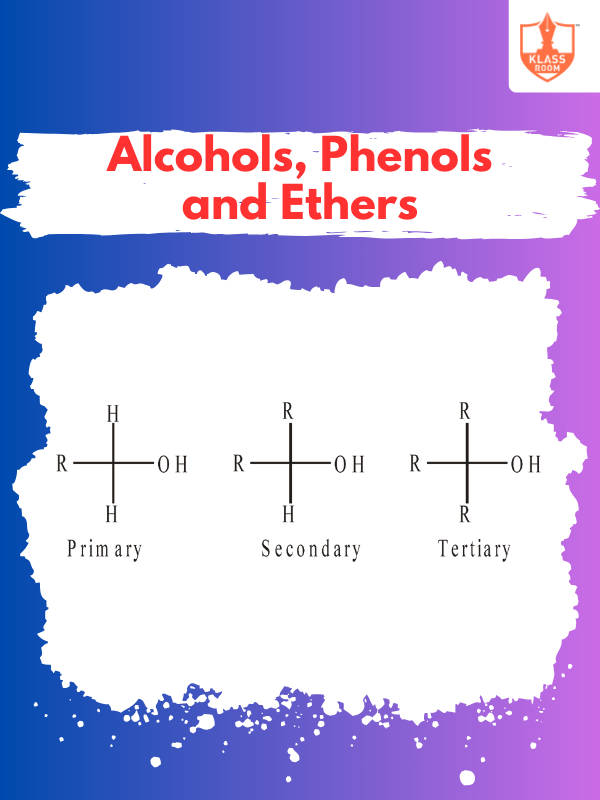
Board: State Board
Stream: Science
Standard: XII
Course: Chemistry
Know More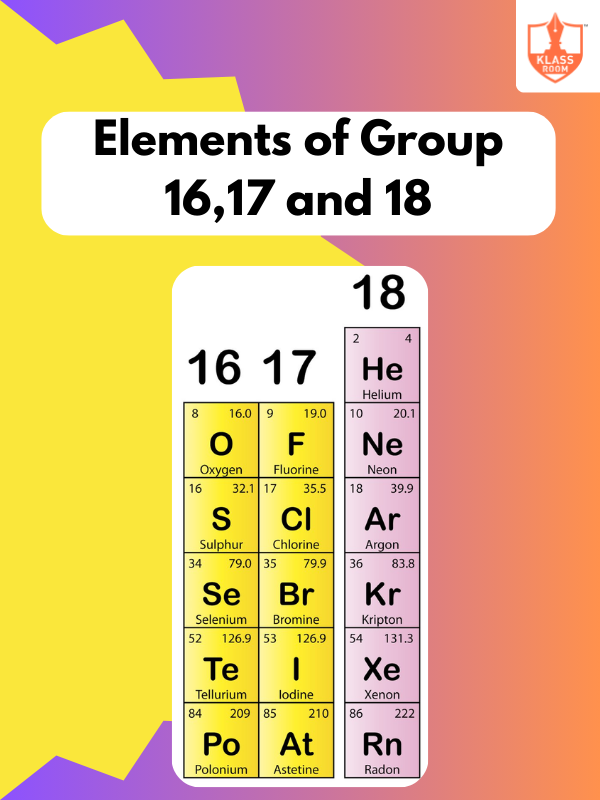
Board: State Board
Stream: Science
Standard: XII
Course: Chemistry
Know More