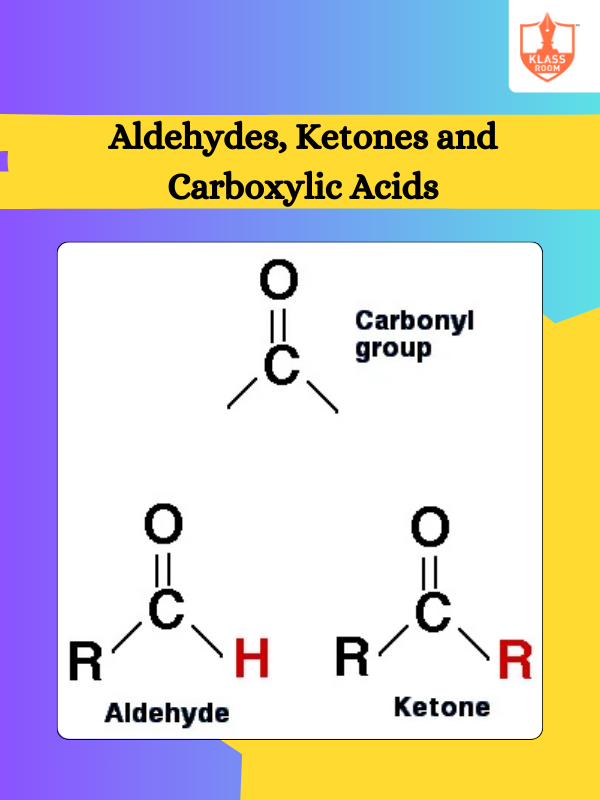
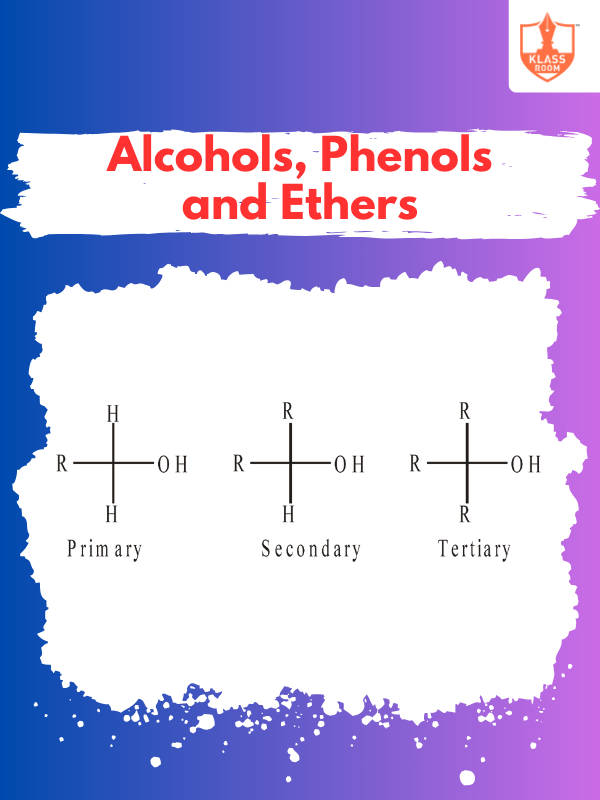
Contents
Periodic Table Overview
Description: The periodic table's objectives include organizing elements by properties, predicted by Newlands's "Law of Octaves" and Lothar Meyer's table, culminating in Mendeleev's and the modern table. Nomenclature and electronic configurations follow.

Configuration & Classification-I
Description: Electronic Configuration II delves into detailed electron arrangements. Group-wise configuration organizes elements based on their respective groups, while block-wise classification arranges elements by electron subshells within the periodic table.

Classification-II
Description: Block-wise classification II categorizes elements by electron subshells. Metals, non-metals, and metalloids are distinguished based on their properties, impacting periodic trends such as atomic radius and ionization energy.

Radius,Enthalpy & Factors
Description: Atomic radii and ionic radii trends show size variations. Lanthanide contraction affects the size of elements. Ionization enthalpy measures energy to remove electrons. Factors like nuclear charge and shielding influence ionization potential.
Energy-Trend
Description: Ionization energy trends indicate energy to remove electrons. Electron gain enthalpy measures energy released when an atom gains an electron. Electronegativity represents an atom's ability to attract electrons.

Properties-Diagonal
Description: Chemical properties exhibit periodic trends based on element groups and periods. Diagonal relationships exist between elements with similar properties diagonally across the periodic table.
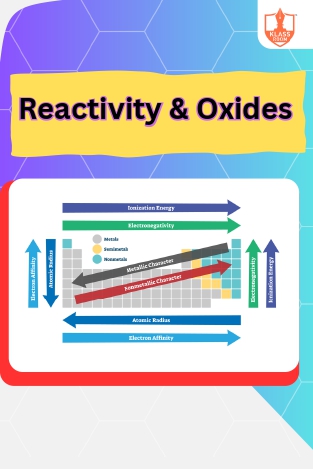
Reactivity & Oxides
Description: Periodic trends govern chemical reactivity, influencing the behavior of elements. Oxides exhibit trends in acidity, basicity, and amphoteric nature based on their position in the periodic table.


.png)